Robert Cullen, DVM / Cats.com
Senvelgo is the brand name for the antidiabetic medication, velagliflozin. In this article you’ll learn how Senvelgo works to treat diabetes in cats, important safety information, and how to decide if Senvelgo is the right choice for your diabetic cat.
Senvelgo for Cats Overview

About Senvelgo for Cats
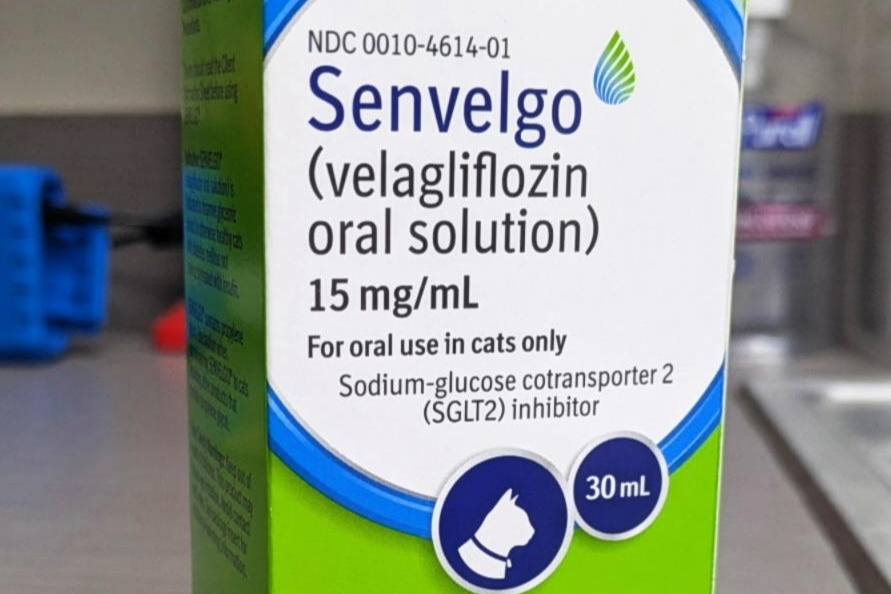
Senvelgo is the brand name for velagliflozin, manufactured by Boehringer Ingelheim Animal Health. Senvelgo is an FDA-approved medication for cats to treat diabetes.
Velagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor. SGLT2 is responsible for reabsorbing a majority of glucose that ends up in the urine. In untreated diabetics, glucose in the bloodstream (blood sugar) reaches a certain threshold before it spills over into the urine.
In diabetics who are either not treated or not well regulated, high blood sugar levels lead to excess sugar in the urine. The excess sugar acts as a diuretic, attracting more water and leading to the excessive urination and thirst commonly seen with diabetics.
SGLT2’s job is to keep sugar in the bloodstream as much as possible by having the kidneys reabsorb glucose before it ends up in urine. This is an important function in healthy pets to help maintain normal blood sugar levels.
SGLT2 inhibitors prevent this from happening, allowing more glucose to end up in the urine. This leads to blood sugar levels being lower.
As the “sodium” part of SGLT2 implies, these medications also block sodium reabsorption, though the significance in cats is unknown.
For SGLT2 inhibitors to be used safely and effectively, a cat must be producing insulin. In most cases of feline diabetes, cats have a form similar to type II in people. Insulin is being produced by the pancreas, but the body has developed resistance to its effects. While rare, it is possible for a cat to have insulin-dependent diabetes mellitus, similar to type I in people. SGLT2 inhibitors cannot be used in these cases.
Senvelgo cannot be used in diabetic cats who have already been treated with insulin. Initiation of Senvelgo alongside insulin administration or in a cat going through withdrawal of insulin has been associated with an increased risk of diabetic ketoacidosis (DKA) and euglycemic DKA.
Dosing Information for Cats
The following information should not be used to replace a veterinary visit or alter your vet’s prescribing information. Always consult with your own vet before starting a medication or adjusting a medication dose for your cat.
Senvelgo oral solution is FDA-approved for use in cats.
The FDA-approved dose is 1 mg/kg given by mouth once daily. It should be given at approximately the same time each day.
While blood sugar monitoring is important for safe use of an SGLT2 inhibitor, the dose of Senvelgo is not adjusted based on blood sugar levels.
The oral solution comes in a concentration of 15mg/ml.
Senvelgo comes with a dosing syringe that has increments based on body weight (not in milliliters). This is to simplify dosing at home. Give your cat the dose that correlates closest to their most recent body weight, using the provided syringe.
But in the event you lose the dosing syringe that comes with Senvelgo, see the dosing chart below for approximate milliliter dosing volumes based on a cat’s weight in pounds.
| 5 lb | 0.15 ml |
| 7 lb | 0.2 ml |
| 8 lb | 0.25 ml |
| 10 lb | 0.3 ml |
| 12 lb | 0.35 ml |
| 14 lb | 0.4 ml |
| 17 lb | 0.5 ml |
| 20 lb | 0.6 ml |
How to Administer Senvelgo to Cats
Senvelgo is an oral liquid solution. Per the manufacturer, it can be given either directly by mouth or it can be given directly on top of a small amount of food. The medication may be applied on top of food, but not mixed in with it.
Because it is given only once a day, a missed dose can be given as soon as possible on the same day.
If a cat vomits within 30 minutes of taking the medication, the dose may be repeated.
If your cat will be having surgery or another procedure where withholding food/fasting is required, the medication should be temporarily discontinued during that time. If you’re not sure if this is necessary, always double check with your veterinary office.
Side Effects of Senvelgo for Cats
The following are the most frequent side effects of Senvelgo and the percentage of cats affected in clinical studies:
- Diarrhea/loose stool (53% of cats)
- Weight loss (44% of cats)
- Vomiting (37% of cats)
In 10%-20% of cats, the following side effects have been reported (in decreasing frequency):
- Increased thirst and urination
- Reduced or absent appetite
- hypersalivation/excessive drooling
- Dehydration
- Elevated blood urea nitrogen (BUN) on lab work
In 1%-10% of cats the following has been reported (in decreasing frequency):
- Lethargy
- Increased appetite
- Urinary tract infection
- Diabetic ketoacidosis (DKA) or euglycemic DKA
- Increased calcium levels
- Inappropriate urination
- Ketones in the urine
- Death
- Elevated liver values on blood work
- Elevated triglyceride levels on blood work
- Elevated phosphorus levels on blood work
- Elevated feline pancreatic lipase (fPL)
- Pancreatitis
- Elevated creatinine on blood work
- Hepatic lipidosis
Senvelgo should be used cautiously in cats with kidney disease. Evaluating kidney function is part of needed monitoring prior to starting this medication and during its use. However, Senvelgo has been used safely in cats with IRIS stage I and II chronic kidney disease.
Most Serious Side Effects of Senvelgo for Cats

Urine test strips like these for use at home can be used to monitor for ketones. Occasional trace ketones may be normal, but consistent findings of higher levels may indicate serious side effects and warrant an urgent visit to the vet. Yaya / Shutterstock.com
Diabetic ketoacidosis (DKA) is the most serious side effect risk of using an SGLT2 inhibitor like Senvelgo. A specific form of DKA called euglycemic DKA can be seen with use of these medications in cats.
When the body cannot utilize blood sugar (which is the main complication from diabetes), a serious condition called diabetic ketoacidosis (DKA) can result. The breakdown of basic sugars and carbohydrates in food results in glucose. Glucose is needed for fueling the body for all of its daily functions. When glucose cannot be used properly by the body, the body will start to break down fat as an energy source instead.
A byproduct of breaking down fat is a ketone. Ketones are okay for short durations when needed. But excessive build-up leads to the body’s chemical state shifting from neutral to more acidic. This is called acidosis. Acidosis causes severe illness.
In general, DKA is the most serious complication of diabetes going undiagnosed or untreated, or not being treated effectively.
DKA is also a serious potential side effect with use of any SGLT2 inhibitor like Senvelgo. SGLT2 inhibitors do not actually assist with glucose use by the body. They instead only keep blood sugar lower by flushing it out in the urine.
To make things more complicated, a specific form of DKA called euglycemic DKA can be seen with SGLT2 use. In most cases of DKA, the blood sugar will be very high. A very high blood sugar combined with other signs of illness can clue a veterinarian into DKA being a concern.
But with SGLT2 inhibitors, the blood sugar may actually be normal (euglycemic) because excess glucose is being pushed into the urine by the medication. This can make diagnosing DKA more challenging.
Euglycemic ketoacidosis could occur in a cat on an SGLT2 inhibitor at any time. It’s very important to closely monitor for signs of concern at home. Signs of DKA can include:
- Reduced or absent appetite
- Vomiting
- Lethargy/weakness
Even if your cat is not showing signs of illness, it’s important to regularly monitor urine and blood serum ketone levels a couple times when first starting Senvelgo, and again every 3-6 months. For full recommendations, see the following section on monitoring.
Ketones detected in the urine above rare trace levels may prompt immediate discontinuation of Senvelgo. Similarly, seeing a blood serum ketone (BHB) level greater than 25 mg/dl prompts discontinuing the medication and prompt initiation of insulin therapy.
Precautions
While some may see treating diabetes with oral medication as easier than insulin injections, it’s important to understand that medications like Senvelgo are not true replacements for insulin therapy. Kirsten McCarthy / Cats.com
SGLT2 inhibitors like Senvelgo may come with less “work” at home compared to giving insulin injections.
But it‘s important to understand there are core differences between SGLT2 inhibitors and insulin therapy. You should not think of Senvelgo as an “easier replacement” for insulin.
When treating a diabetic cat with insulin, the goal is essentially to replicate the body’s normal process of producing insulin to carry glucose into cells where it can be used. As discussed above with euglycemic DKA, SGLT2 inhibitors do not actually perform the same function.
Cats being treated with insulin may be able to go into diabetic remission. This is where the body returns to being able to regulate blood sugar on its own without the need for insulin injections. Approximately 50% of cats on insulin therapy may go into remission. This is best achieved within 6 months of diagnosis. Diabetes may recur, but more than half of cats that go into remission will remain in remission.
Cats on SGLT2 inhibitors will not have the opportunity to go into remission. This is important to keep in mind when considering what approach to take with your kitty if diabetes is newly diagnosed.
Monitoring a Cat on Senvelgo
The following monitoring parameters are recommended prior to starting a cat on Senvelgo:
- Patient history and physical exam (these should include activity level, appetite, food/water intake, and body weight)
- Complete blood count (CBC)
- Serum chemistry to evaluate liver and kidney function, cholesterol and triglyceride levels, and electrolytes (including calcium)
- Fructosamine (representing average blood glucose levels over the previous couple of weeks)
- Feline pancreatic lipase (fPL) to screen for pancreatitis (an ultrasound may also be needed)
- Urinalysis to screen for urinary tract infection and the presence of ketones)
- Blood or serum ketones (beta-hydroxybutyrate or BHB)
Within the first 2 to 3 days of starting Senvelgo, the following should be evaluated:
- Urine ketones
- Blood serum ketones (BHB)
At about 1 week after starting Senvelgo, the following should be evaluated:
- Glycemic control with either an 8 hour blood glucose curve and/or a serum fructosamine
- Activity level, appetite, food and water appetite
- Hydration status
- Body weight
- Urine ketones
- Blood/serum ketones (BHB)
At the 4th week after starting Senvelgo, the following should be evaluated:
- Blood glucose control with an 8 hour blood glucose curve and/or fructosamine level
- Physical examination
- Body weight
The following should be evaluated routinely during treatment with Senvelgo (approximately every 3-6 months):
- Activity level
- Body weight
- Appetite/food and water intake
- Urine output
- Hydration status
- Lab work to check liver function, kidney function, cholesterol, triglycerides, and electrolytes (including calcium)
- A blood glucose curve and/or a serum fructosamine
- Urinalysis to monitor for urine ketones and urinary tract infection
Because Senvelgo’s method of action is to increase urine glucose output, monitoring using urine glucose on a urine dipstick is of limited value.
Overdose and Emergencies
For the most part, overdoses or acute toxicity with Senvelgo will lead to extensions of the most common side effects. These include vomiting and diarrhea.
But these could also be seen with a cat experiencing diabetic ketoacidosis (DKA) or euglycemic DKA. Any signs of digestive upset, reduced appetite, or concerns for an overdose of Senvelgo should prompt you to immediately contact one or more of the following for further advice:
- Your veterinarian
- ASPCA Animal Poison Control Center (1-888-426-4435)
- Pet Poison Helpline (1-855-764-7661)
Potential Drug Interactions With Senvelgo
Senvelgo is still a relatively new medication at the time of this article’s publication. All medication interactions with Senvelgo are not known. The following list represents interactions that have either been reported or are theoretically possible.
Unless specifically indicated, this list does not mean the following medications cannot be used with Senvelgo. Weigh the potential risks and benefits. Make sure to discuss all medications your cat is receiving with your vet.
- Angiotensin-converting enzyme (ACE) inhibitors (enalapril, benazepril): may increase low blood sugar effects. May increase risk for low blood pressure and acute kidney injury, especially in dehydrated cats.
- Antihypertensive agents (amlodipine, telmisartan): may increase risk for low blood pressure and acute kidney injury, especially in dehydrated cats.
- Beta blockers (atenolol, propranolol): may increase low blood sugar effects. May increase risk for low blood pressure and acute kidney injury, especially in dehydrated cats.
- Corticosteroids (prednisolone): may decrease the blood sugar effect lowering of Senvelgo
- Diuretics (furosemide/Lasix): added low blood pressure and diuretic effects may occur.
- Estrogens: may decrease the blood sugar lowering effect of Senvelgo
- Fluoroquinolones (marbofloxacin, pradofloxacin): may increase the blood sugar lowering effect
- Insulin: serious adverse effects are more likely to occur in cats previously treated with, or currently receiving, insulin injections. Senvelgo should not be used in cats who have previously received insulin injections or are currently on insulin therapy.
- NSAIDs (robenacoxib, meloxicam): Acute kidney injury risk is increased in humans experiencing dehydration while taking an SGLT2 inhibitor
- Progestogens: may decrease the blood sugar lowering effect
- Selective serotonin reuptake inhibitors (SSRIs) (fluoxetine): may increase the blood sugar lowering effect.
- Sulfonylureas (glipizide): additional blood sugar lowering effects may occur. Use of glipizide with SGLT2 inhibitors has not been evaluated in cats.
How to Store Senvelgo
According to the manufacturer, Senvelgo can be stored at or below 77 degrees F (25 degrees C). The solution will still remain stable with brief excursions up to 104 degrees F (40 degrees C).
Senvelgo should be used within 6 months of opening the bottle.
Drug Dosing Disclaimer: We are only able to provide doses for medications that are FDA approved for use in cats and only as the label guidelines dictate. For medications that are used off-label we can only provide guidelines and safety information for use. Safe and appropriate dosing for off-label medications can only be determined by a primary care veterinarian.
We encourage you to work with your veterinarian to determine if a particular medication is appropriate for your cat. Changing or adjusting a dose for your cat on your own without consulting with a veterinarian can carry risk. We do not encourage use of medications prescribed for human use in pets without first consulting with a primary care veterinarian.
-
A Budde, J., & A McCluskey, D. (2023). Velagliflozin [Professional app]. In Plumb’s Veterinary Drug Handbook (10th ed.). Wiley Blackwell.
-
U.S. Food and Drug Administration. (2024, April 3). Dear Veterinarian Letter regarding important safety conditions associated with the use of Senvelgo (velagliflozin oral solution) for improving glycemic control in certain cats with diabetes mellitus. U.S. Food And Drug Administration.
-
Williams, K. (n.d.). Diabetic Ketoacidosis in Cats. VCA Animal Hospitals.
-
Llera, R., & Buzhardt, L. (n.d.). Diabetic Remission in Cats. VCA Animal Hospitals.
-
Gottlieb, S., Rand, J. S., & Anderson, S. T. (2024). Frequency of diabetic remission, predictors of remission and survival in cats using a low-cost, moderate-intensity, home-monitoring protocol and twice-daily glargine. Journal of Feline Medicine and Surgery, 26(4). https://doi.org/10.1177/1098612x241232546